The spent biosorbent generated after multi-metal (Ni(II), Co(II), Zn(II) and Cd(II)) biosorption using dried activated waste tannery sludge were applied for glyphosate removal. The removal efficiency could be enhanced from 26.18 % to >92% after increase in the multi-metal loading on the sludge. The process showed a gradual removal at wide pH range within 24-28 h of contact time. Similar biosorption efficiency (91.7%) was observed for treatment of simulated agricultural wastewater.
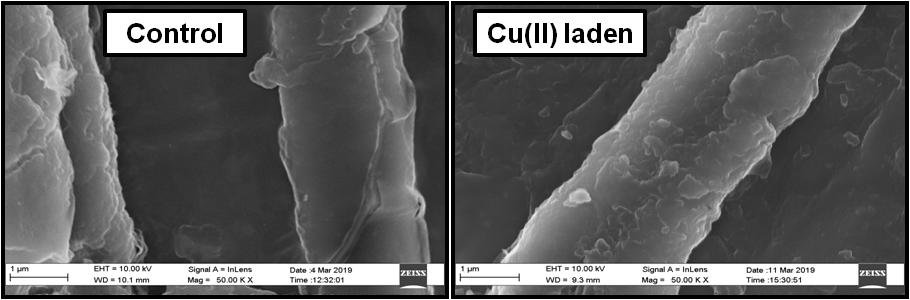
The biochar developed from jute industry waste commonly known as jute caddies was proposed as low cost, easy to prepared, efficient and suitable adsorbent for removal of Cu(II) metal ions from synthetic as well as from simulated jute industry wastewater. Feasibility study for real effluent treatment exhibited comparable adsorption efficiency as the synthetic Cu(II) solution. The studies showed about 98.74% removal at pH 5.1 and 97.15% removal of Cu(II) at without adjusted pH of the Cu(II) simulated jute industry wastewater. The maximum adsorption capacity for Cu(II) ions onto the developed biochar was found 588.25mgg-1 at original pH of the metal solution within 4h of the contact time.
Last Updated on December 4, 2020