English | हिन्दी

সিএসআইআর-কেন্দ্রীয় কাঁচ ও সেরামিক গবেষণা সংস্থা
सीएसआईआर-केंद्रीय काँच एवं सिरामिक अनुसंधान संस्थान
CSIR-Central Glass & Ceramic Research Institute

Bioceramics & Coating
Overview

We strongly believe in collaborative research and through effective alliance programs, that include close association of scientists, technologists, surgeons and industries, we actively work on research project that translate into effective healthcare products. With this ideology our research group has developed ceramic femoral head articulating against standard polyethylene of hip joint prosthesis. This product has been clinically tested followed by commercialization. Currently, we are working on new ceramic matrix composite for ceramic-on-ceramic articulating system for Total Hip Arthroplasty. To improve osseointegration of metallic implants we have developed bioactive hydroxyapatite (HAp) coating using plasma spraying and these coatings have been tested clinically. Other commercial biomedical products developed by the division include bioglass coatings, bioactive ceramic scaffolds with controlled porosity characteristics, calcium phosphate granules (single and biphasic) for dental and orthopaedic applications and hydroxyapatite based integrated orbital implant. Other important materials for biomedical applications that are currently under development include ceramic composites, hard coatings, bioglass and nanopowder for drug delivery. In addition to healthcare section, our division is developing ceramic components such as high-temperature, non-shrinkable ceramic potting material, freestanding diamond windows and diamond rods for supports in helix traveling-wave tube (TWT), as a part of India’s contribution towards International Thermonuclear Experimental Reactor (ITER) program.
The division has developed 11 technologies, commercialized 9 technologies and received two best technology awards from Government of India. We currently hold 23 patents including 2 USA and Japan patents. Currently, the division has research funding of ₹ 489 million (USD 7.69 million) during 2012 – 2018 and the divisional members include 7 scientists, 8 technical staff, 17 students (10 Ph.D.; 2 M.Tech; 5 Project staff).
Areas of Research
Due to our extensive collaboration throughout the country and abroad, our research activities include:
- Orthopaedic, dental and maxillofacial implants/materials
- Ceramics and ceramic composites for hip and knee prosthesis.
- Metallic and ceramic implants for finger joints, oncology and spine replacement.
- Bioresorbable materials for bone replacement.
- Bioactive glass and ceramic based cements/materials.
- Tissue engineering & drug delivery
- Bioactive, porous materials for orbital implants and wound dressing applications.
- Tailored/designed scaffolds for onsite drug delivery.
- Nanoceramic based inorganic drug delivery systems for cancer therapy.
- Reconstructive and Trauma materials
- Tailored/designed scaffolds for orthopaedic, cosmetic, cranioplasty reconstruction.
- Patient specific and customized implants via additive manufacturing.
- Coatings
- Hard, wear resistance and biocompatible metallic, ceramic and composite coatings for load-bearing implants.
- Bioactive, osteoconductive and osteoinductive coatings on bioinert medical materials.
- Porous coatings for bone tissue ingrowth.
- Microwave plasma chemical vapor deposition of diamond coatings.
- Other materials and areas
- Ceramic and polycrystalline diamond materials for electron tubes.
- Ceramic joining.
- Microwave processing of ceramic materials.
- Unconventional cancer and wound treatment.
Collaboration
India
- Indian Institute of Technology, Madras.
- Indian Institute of Technology, Kanpur.
- Indian Institute of Technology, Delhi.
- Indian Institute of Science, Bangalore.
- Indian Institute of Technology, Kharagpur.
- Vellore Institute of Technology, Vellore.
- National Institute of Technology, Suratkal.
- Malaviya National Institute of Technology, Jaipur.
- West Bengal U of Animal & Fishery Sci, Kolkata.
- Sancheti Inst. for Orthop. & Rehabilitation, Pune.
- Tata Memorial Hospital, Mumbai.
- Jubilant Kalpataru Hospital, Barasat.
- Disha Eye Hospitals and Research Centre (P) Ltd., Barrackpore, West Bengal.
- Sri Sankaradeva Nethralaya, Guwahati.
- Saijyothi Eye Institute, Secunderabad.
- Sir Ganga Ram Hospital, New Delhi.
- Wockhardt Medical Centre, Kolkata.
- R.G. Kar Medical College & Hospital, Kolkata.
- Burdwan Medical College & Hospital, Burdwan.
- Calcutta Medical College & Hospital, Kolkata.
- Regional Institute of Ophthalmology, National Medical College and Hospital, Kolkata.
- N.R.S. Medical College and Hospital, Kolkata.
- Sambhu Nath Pandit Hospital, Kolkata.
- Calcutta Medical Research Institute, Kolkata.
- All India Institute of Medical Sciences, New Delhi.
- Maulana Azad Dental College & Hospital, New Delhi.
- Dr. Rafi Ahmed Dental College & Hospital, Kolkata.
- M/s General Surgical Company (India) Pvt Ltd., Chennai.
- M/s Orthotech, Gujarat.
- M/s Mu Biomagg, Gujarat.
Foreign
- Medical University of Vienna, Austria.
- Washington State University, USA.
- University of Louisville, USA.
- A.M. Prokhorov Gen. Phys. Inst., Russia.
- University of Aveiro, Portugal.
- University of Turku, Finland.
- Abo Academy University, Finland.
- Univ. of Vigo, Spain.
- Mindanao State Univ., Philippines.
- Universität Bremen, Germany.
Technology Developed
Technologies available for licensing
 1. Ceramic based total hip prosthesis (ceramic-on-PE). |
 2.Plasma spray hydroxyapatite coating on metallic biomedical implants. |
|
 3. Manufacture of bioactive ceramic scaffolds with controlled porosity characteristics. |
 4. Calcium phosphate granules (single and biphasic) for dental and orthopaedic applications. |
|
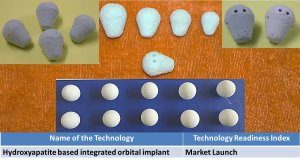 5. Hydroxyapatite based integrated orbital implant. |
 6. High-temperature protective coatings for automobile heater plugs. |
|
 7. Glass lining coatings. |
 8. High-temperature protective coatings. |
|
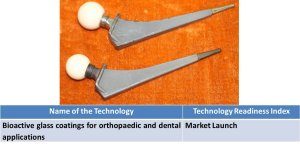 9. Bioactive glass coatings for orthopaedic and dental applications. |
 10. Plasma spray grade hydroxyapatite powder preparation. |
|
 11. Injectable, biodegradable bone cement composite with/without drugs. |
Technologies under development
| Sl. No. | Title | Technology Readiness Index |
| 1 | An inorganic/ceramic based antacid for instant relief and sustained buffering action. | Lab Validation |
| 2 | Novel HAp coated bone plates for bone plate fracture | Lab validation |
| 3 | Hydroxyapatite-bioactive glass composite for spinal and dental applications | Lab validation |
| 4 | Laser assisted deposition of in-situ synthesized TiB-TiN reinforced Ti alloy composites for biomedical and other applications. | Prototype |
| 4 | Metacarpophalangeal joint replacement prosthesis | Prototype (under clinical trials) |
| 6 | Alternative adjuvant for anticancer drugs | Proof of Concept |
| 7 | Unconventional cancer treatment without drugs | Proof of Concept |
| 8 | New ceramic composite composition for total hip prosthesis | Concept Definition |
| 9 | Non-invasive acceleration of tissue-artificial implant integration | Concept Definition |
| 10 | Porous metal coatings on metals and plastics for osseointegration | Concept Definition |
| 11 | Novel degradable Mg alloys with tailored resorption | Concept Definition |
| 12 | Patient specific customized implants | Concept Definition |
| 13 | Extendable implants for juvenile bone cancer patients | Concept Definition |
| 14 | Electrospun fiber composites for bone or skin wound healing | Concept Definition |
Technology Readiness Index: Idea, Concept Definition, Proof of Concept, Prototype, Lab Validation, Technology Development, Technology Demonstration, Technology Integrated, Market Launch.
Projects
| Sl. No | Title of the Project | Duration | Project Category | Funding Agency | Principal Investigator | Co-Investigator(s) & members |
| 1 | Oxidation and hot corrosion study on functionally graded multilayer thermal barrier coating for gas turbine applications | 2018-2021 | GAP | AR&DB | Dr. Sumana Ghosh | Dr. Mitun Das |
| 2 | Additive Manufacturing by Laser Metal Deposition | 2018-2019 | OLP | TATA Steel | Dr. V. K. Balla | Dr. Mitun Das Dr. Subhadip Bodhak |
| 3 | Wear resistant ceramics for cutting & milling operation: Process optimization of SiAlON-WC composites for rock drilling application | 2018-2020 | FTT | CSIR | Dr. S. Bandyopadhyay | Dr. S. Sarkar |
| 4 | Sugar-Glass Nanoparticles Encapsulated Multifunctional Nanofibrous Patch For Intervertebral Disc Regeneration | 2018-23 | GAP | DBT (Ramalingaswami Reentry Fellowship) | Dr. Subhadip Bodhak | None |
| 5 | Preparation of high Heat Resistant CG-B-55A enamel frit for application in MiG series aero-engines parts | 2017-18 | SSP | HAL | Dr. Sumana Ghosh | Dr. J. Chakraborty Member: Dr. S. Sarkar |
| 6 | Preparation of high Heat Resistant CB-ABK-13 enamel frit for application in MiG series aero-engines parts | 2017-18 | SSP | HAL | Dr. Sumana Ghosh | Dr. S. Sarkar Member: Dr. J. Chakraborty |
| 7 | Microstructurally designed in-situ toughened metallic and ceramic matrix composites for total hip arthroplasty | 2016-19 | GAP | DBT | Dr. V. K. Balla | Dr. M. Das, S. Gangadharan Member: Dr. S. Bodhok, Dr. B. Kundu, A. C Das, D. Bhattacharya, P. Patra |
| 8 | Laser based additive manufacturing of in situ formed discontinuously reinforced Ti-TiB composite structures | 2016-19 | GAP | AR&DB | Dr. M. Das | Dr. V. K. Balla |
| 9 | Prototype Development of in vivo tested HDPE based biocompatible composite with HA/Al2O3 Ceramic Fillers as Acetabular Cup for Total Hip Replacement | 2015-20 | GAP | DBT | Dr. B. Basu (IISc), Dr. V. K. Balla (CGCRI) |
Dr. B. Kundu Member: Dr. M. Das Dr. S. Bodhak |
| 10 | Development of hydroxyapatite based modified integrated orbital implant with superior motility and its clinical trial | 2013-16 | SBMT | CSIR-CGCRI | B. Kundu | V. K. Balla |
| 11 | Development of Novel Biomedical Implants with Enhanced Reliability | 2013-16 | DST | CSIR-CGRI | A.K. Mukhopadhyay | V.K. Balla |
| 12 | The prospect of cancer therapy using an inorganic nano conjugate comprising microRNA-34 family | 2017-20 | GAP | DST | J. Chakraborty (CSIR-CGCRI) | Members: S.Ghosh, S.Bodhak, S.Sarkar |
| 1 | Development of large size polycrystalline CVD diamond material for optical windows and support rods in high power microwave tubes | 2015-18 | DST | V.K. Balla (CGCRI), PI from Russia |
A.K. Mallik | |
| 2 | Electron microscopy study of the degradation kinetics of porous bioactive glass based novel drug eluting implants (coating/3D scaffolds) as a function of hard tissue regeneration for treatment of osteoporotic fractures in elderly patients | 2015-18 | GAP | DST | J. Chakraborty (CSIR-CGCRI) Dr. Nina Daneu (Jozef Stefan Institute, Ljubljana, Slovenia) |
B. Karmakar |
| 3 | Borate based bioactive glass nano-fibers for topical application for treatment of non-healing diabetic wounds | 2017-19 | GAP | DST | J. Chakraborty (CGCRI) Prof. Mona Marei, Head, Tissue Engineering Laboratories, Faculty of Dentistry, University of Alexandria, Egypt, |
Dr. Swapan Kr. Ghosh (PMD) |
Completed Projects
| Sl. No | Title of the Project | Duration | Funding Agency | Participating Inst./Lab. | Principal Investigator | Co-Investigator(s) |
| 1 | SiAlON inserts for High Speed Cutting of Hard Materials | 2016-18 | FTT | CSIR | Dr. S. Bandyopadhyay | Member: Dr. S. Sarkar |
| 2 | Development of novel ion doped hydroxyapatite by spray drying method and its utilization for plasma spray coating on medical implants with/without ion doping | 2016-18 | FTT | CSIR | Dr. B. Kundu | Dr. V. K. Balla Members: D. Bhatterchery, S. Majumder |
| 3 | Development of novel CSIR technology for manufacturing tailored and patient-specific bio-ceramic implants and biomedical devices at affordable cost (BIOCERAM) | 2012-17 | CSIR | Dr. V.K. Balla (coordinator) | S. Gangadharan, Dr. J. Chakraborty, Dr. B. Kundu, Dr. M. Das | |
| 4 | Structure of gradient nanocomposites: Interaction of bioactive glasses with nanoparticles and polymers (MoreBAGS) (GAP 0614) | 2012-16 | DST | Dr. G. De (CGCRI), Prof. Pekka Vallittu (University of Turku, Finland) and Dr. Leena Hupa (Åbo Akademi Univ.) | Dr. B. Kundu, Dr. V.K. Balla | |
| 5 | Very High Power Microwave Tubes: Design and Development Capabilities (MTDDC) | 2012-16 | CSIR | Dr. S. Ghosh | A.K. Mallick | |
| 6 | Comparative study of conventional processing with microwave processing of bioactive glass-ceramic coating on Ti6Al4V substrate for biomedical applications | 2013-16 | DST | Dr. S. Ghosh | ||
| 7 | Up-gradation of gamma radiation facility and periodic standardization for optimal RISUG production | 2015-17 | ICMR | B. Kundu | V.K. Balla | |
| 8 | Development of bio-ceramic based implants for rehabilitation | 2008-13 | CSIR | CSIR-CGCRI; SIOR & PHS, Pune | D. Basu & V.K. Balla | M. Das |
| 9 | Design and fabrication capabilities for very high power microwave tubes | 2007-12 | CSIR | CSIR-CGCRI & CSIR-CEERI | D. Basu | S. Datta |
| 10 | New insights in cancer biology identification of novel targets and development of target based molecular medicine | 2007-12 | CSIR | CSIR-CGCRI & CSIR-IICB | M.K. Sinha | B. Kundu |
| 11 | Nanomaterials and nanodevices in health and disease | 2007-12 | CSIR | CSIR-CGCRI & CSIR-CCMB | D. Basu | M.K. Sinha, Jui Chakraborty |
| 12 | Development of bioactive ceramic coating on orthopedic implants (metallic) for sustained, localized delivery of biophosphonates to improve fixation | 2009-11 | DST | CSIR-CGCRI | Jui Chakraborty | D. Basu |
| 13 | Synthesis and characterization of NZP based material for immobilization of radioactive waste | 2008-11 | BRNS (DAE) | CSIR-CGCRI | D. Basu | A. Das Gupta |
| 14 | Development of Ceramic-based Implantable Delivery System for Sustained Release of the Drugs for the Treatment of Osteomyelitis in Human Patients | 2007-10 | DST | CSIR-CGCRI, WBUAFS & R.G. Kar Medical College | B. Kundu | D. Basu |
| 15 | Fabrication of Yttria Doped Thoria (YDT) Thimbles and glass soldering them to Fe-Ni alloy component towards development of oxygen sensor for use in sodium coolant of fast breeder reactors | 2006-09 | IGCAR | CSIR-CGCRI | A. Das Gupta | D. Basu |
| 16 | Micromechanical characterization of hydroxyapatite coated metallic implants | 2006-09 | DST | CSIR-CGCRI | D. Basu | A.K. Mukhopadhyay |
| 17 | Development of selected medical implants (NMITLI) | 2005-08 | CSIR | CSIR-CGCRI | D. Basu | M.K. Sinha |
| 18 | Development of Glass-Ceramic Coatings for High Temperature Protection of Titanium Aluminide Alloys | 2005-08 | ARDB | CSIR-CGCRI | S. Datta | D. Basu |
| 19 | Multi-centred clinical trials for human implantation of bio-active integrated orbital implants | 2005-08 | ICMR | CSIR-CGCRI | D. Basu | B. Kundu |
| 20 | Development of bio-active bio-glass compositions with varied amount of calcium phosphate crystals to be used as coating on titanium and stainless steel implants | 2005-08 | DST | CSIR-CGCRI | S. Datta | D. Basu |
| 21 | Electron Tube Technologies for Large Scale Application | 2002-07 | CSIR | CSIR-CGCRI & CSIR-CEERI | D. Basu | S. Datta |
| 22 | Novel Synthetic Route for Biomaterials and their Applications | 2002-07 | CSIR | CSIR-CGCRI | D. Basu | M.K. Sinha |
| 23 | Developing Capabilities in Advanced Manufacturing Technology | 2002-07 | CSIR | CSIR-CGCRI & CSIR-CMERI | D. Basu | S. Datta |
| 24 | Development of plasma sprayed hydroxyapatite coated metallic implants for orthopaedic application | 2003-06 | DST | CSIR-CGCRI | D. Basu | M.K. Sinha |
| 25 | Development of metallized alumina substrates for fabrication of thermoelectric coolers | 2003-05 | SSPL (DRDO) | CSIR-CGCRI | S. Datta | D. Basu |
| 26 | Development of Bio-active Integrated Orbital Implant | 2001-04 | SBMT | CSIR-CGCRI | D. Basu | M.K. Sinha |
| 1 | Next Generation Functional Implants via Laser Engineering Net Shaping (LENS™) | 2012-15 | CSIR | CSIR-CGCRI, W. M. Keck Biomedical Materials Research Lab, Washington State University, USA. | V.K. Balla | Mitun Das |
| 2 | Development of functionally graded patient specific orthopedic implants by rapid prototyping technique and their evaluation in-vitro & in-vivo | 2009-12 | DST | CSIR-CGCRI; Washington State University, USA | D. Basu | M. Das |
| 3 | Development of Thermally Sprayed Ceramic Based Coatings | 2007-10 | TIFAC, DST | CSIR-CGCRI; ARCI, Hyderabad; School of Mechanical & Production Engg., Singapore; Advanced Materials Research Centre, Malayasia; & Indonesian Institute of Sciences | D. Basu | M.K. Sinha |
| 4 | Study of Remodeling of bone-ceramic interface to assess cell growth kinetics as a function of composition and morphological modification of ceramic implant | 2007-10 | DST | CSIR-CGCRI; Dept. of Nanostructured Materials, Ljubljana, Slovenia | D. Basu | M.K. Sinha |
| 5 | Development of ceramic-based implantable delivery system for sustained release of the drugs for treatment of osteomyelitis and diabetes | 2007-10 | DST | CSIR-CGCRI; University of Aveiro, Portugal | D. Basu | B. Kundu |
| 6 | Development of SiAlON ceramics for tribological applications | 2006-09 | CSIR | CSIR-CGCRI; Turkey | D. Basu | S. Bandyopadhyay |
Collaboration
India
- Indian Institute of Technology, Madras.
- Indian Institute of Technology, Kanpur.
- Indian Institute of Technology, Delhi.
- Indian Institute of Science, Bangalore.
- Indian Institute of Technology, Kharagpur.
- Vellore Institute of Technology, Vellore.
- National Institute of Technology, Suratkal.
- Malaviya National Institute of Technology, Jaipur.
- West Bengal U of Animal & Fishery Sci, Kolkata.
- Sancheti Inst. for Orthop. & Rehabilitation, Pune.
- Tata Memorial Hospital, Mumbai.
- Jubilant Kalpataru Hospital, Barasat.
- Disha Eye Hospitals and Research Centre (P) Ltd., Barrackpore, West Bengal.
- Sri Sankaradeva Nethralaya, Guwahati.
- Saijyothi Eye Institute, Secunderabad.
- Sir Ganga Ram Hospital, New Delhi.
- Wockhardt Medical Centre, Kolkata.
- R.G. Kar Medical College & Hospital, Kolkata.
- Burdwan Medical College & Hospital, Burdwan.
- Calcutta Medical College & Hospital, Kolkata.
- Regional Institute of Ophthalmology, National Medical College and Hospital, Kolkata.
- N.R.S. Medical College and Hospital, Kolkata.
- Sambhu Nath Pandit Hospital, Kolkata.
- Calcutta Medical Research Institute, Kolkata.
- All India Institute of Medical Sciences, New Delhi.
- Maulana Azad Dental College & Hospital, New Delhi.
- Dr. Rafi Ahmed Dental College & Hospital, Kolkata.
- M/s General Surgical Company (India) Pvt Ltd., Chennai.
- M/s Orthotech, Gujarat.
- M/s Mu Biomagg, Gujarat.
Foreign
- Medical University of Vienna, Austria.
- Washington State University, USA.
- University of Louisville, USA.
- A.M. Prokhorov Gen. Phys. Inst. , Russia.
- University of Aveiro, Portugal.
- University of Turku, Finland.
- Abo Academy University, Finland.
- Univ. of Vigo, Spain.
- Mindanao State Univ., Philippines.
- Universität Bremen, Germany.
Divisional Facilities

Laser based additive manufacturing facility (MR-7, Optomec Inc., USA)
Laser Engineered Net Shaping (LENSTM) is an laser based additive manufacturing facility that uses metal powder to create net shape functional parts from CAD files for variety of applications. Our system uses 500W fiber laser with 0.5 mm beam diameter. The process begins with creating a molten metal pool on a substrate fixed to a X-Y CNC table. A predetermined amount of powder is fed into the liquid metal pool and as the substrate moves a thin line of metal strongly bonded to substrate will be created. A cross section is built by overlapping these thin lines. The deposition head moves up once the layer is deposited and process is repeated until the part represented in CAD file is fabricated. Parts with fully dense or tailored internal architecture or porosity can be built. We have successfully processed Ti, Ti6Al4V, stainless steels, NiTi alloy, intermetallics, and metal matrix composites.
Air plasma spraying facility (9M, Sulzer Metco.)
Our 80 kW air plasma spray system consists of plasma spray gun, spray controlling unit, power supply unit, distribution unit, powder feeder unit, etc. The unit can deposit variety of materials creating sound coatings of different substrates for biomedical and other engineering applications. We have successfully deposited hydroxyapatite, tri-calcium phosphate and/or bioactive glass coatings on metallic biomedical implants/ devices with different designs. Other types of coatings such as in-situ metal matrix coatings and porous metal coatings are currently under development for bone implant applications.

Microwave plasma chemical vapor deposition system (DT1800, Lambda Technologies Inc. USA)

One MPCVD reactor with 915 MHz and 15 kW microwave generator has been installed as a part of 11th five year plan period. We have extensively used this system for growing free standing diamond for microwave application, which is a part of ITR project. We have successfully demonstrated fabrication capability up to 60 mm diameter and 0.6 – 0.8 mm thick polycrystalline diamond discs for this application.
Chemo-mechanical (CMP) polishing (CETR, USA)
This apparatus has been specifically customized to polish hard materials such as CVD diamond developed in our laboratory. The instrument has capability to polish at varying process parameters such as normal load, sliding speed, travel speed and has provision to dispense specified amount of polishing fluids to control rate of polishing.
Using this process we could reduce the surface roughness of CVD diamond from ~ 6 µm to 200 nm.
Hot and cold isostatic presses (QIH-6, Avure Technologies Inc., USA; EPSI, Belgium)
Both these presses are primarily used to compact ceramic materials. The cold isostatic press is primarily used to achieve high green density of ceramic materials using pressures up to 400 MPa. On the other hand, the hot isostatic press is being used to combine pressing and sintering in one step. Further, we use this process to achieve highest possible sintered density in ceramics for demanding applications such as medical applications. The hot isostatic pressing capabilities include up 2000?C temperature and 200 MPa pressure.

Hip and knee joint simulators (8511, Instron, USA; ProSIM, Simsol Ltd., UK)
In vitro tribological testing using implant simulators is an important aspect in pre-clinical validation of articulating biomaterials as these tests simulate in vivo physiological conditions (loading and movements) thereby providing close in vivo wear performance of articulating biomaterials. It was observed that the wear particles retrieved from sliding wear testing were considerably larger (100-500 nm) than those obtained from hip simulator testing (5-70 nm) and the discrepancy is primarily due to the differences in wear test conditions such as sample geometry, type of loading, sliding, etc. Since the periprosthetic tissue reactions and osteolysis surrounding the hip and the knee implants are linked to the total wear particle characteristics such as volume, particle size, shape and composition tribological testing using joint simulators assumes significant importance. Therefore, we perform tribological evaluations of our materials and designs using these implant simulators to validate their performance.

Gamma-ray sterilization facility (GC 5000, BRIT, BARC)

This facility is being used for sterilization of implants for clinical trials and also to study the effect of gamma ray on the physical and mechanical properties of the new materials that are being developed at our division. Our facility has a Co60 source which works both at low and high doses, e.g., 10 kGy for food processing; 25 kGy for regular disinfection of metal/ ceramic/ polymer implants.
In vitro tissue culture laboratory
This laboratory is equipped with the following:
Class II Type A2 Biosafety Cabinet (ESCO):

Our biological safety cabinet is latest generation of energy efficient biosafety cabinet and utilizes ULPA filters for contamination protection. The exterior is coated with Isocide™ powder coating to prevent microbial/bacterial growth. All types of our in vitro cell culture experiments will be performed in this biosafety cabinet.
CO2 incubator: This incubator is being used in our research to grow and maintain cell cultures. Our fields of application include tissue engineering, in vitro culturing, cancer research, and other cell research.
Tissue culture inverted microscope: This inverted microscope is used for biological applications such as to view tissue culture specimens grown in flask.
Plate reader: The reader is used to detect biological, chemical or physical activities of cells. We use 96-well plate with a typical media volume of 100-200 µL/well. The absorbance is detected for quantitative assays such as protein/enzyme activity assays (MTT assay for cell viability). The amount of transmitted light is related to the concentration of desired molecule.
Gel documentation unit: The gel doc imager is an automated and time-saving system to image and analyze electrophoresis gels. The data can be viewed, modified and reported using the Image Lab software. This technique identify and image cellular protein/nucleic acids in a given state, e.g., by application of a new formulation/therapeutic agent etc.
In vitro tribological and electrochemical testing facilities (Nanovea, USA; Bio-Logic Science Instruments SAS, France)

Our tribological testing system is equipped with rotating and pseudo linear reciprocating type wear testing modules. The test setup also contains accessories to carry out tests in physiological fluid with precise temperature control to represent biological environment in the body. Ball-on-disc or pin-on-disc wear testing can be performed using tribometer with ? 3 mm or ? 6 mm pins rubbing against test samples. Normal loads up to 40N can be used for tests.

Electrochemical testing setup has potentiostat, galvanostat, electrometer and frequency response analyzer (FRA) designed to perform several electrochemical research experiments. We extensively use this instrument for electrochemical performance evaluation, such potentiodynamic, electrochemical impedance analysis, of variety of materials and coatings we develop for biomedical applications.
Optical microscope with fluorescence attachment (Olympus, Japan)

This microscope has Bright Field (BF), Differential Interference Contrast (DIC) and Reflected Fluorescence observation facilities with Image Analysis capabilities. The microscope is capable to perform microstructural investigation/imaging for different materials as well as biological samples with Fluorescence attachment.
Compact scanning electron microscope with composition analysis (EDX) (Phenom proX, Phenom-World B.V., Netherlands)

This is compact/desktop SEM with BSE detector with composition analysis capability (point, line scan and mapping) using EDX detector. The magnification can go up to 40,000X. We have three types of samples holder to image microstructures and composition analysis of conductive, non-conductive and mounted samples.
General facilities
- Hydraulic and mechanical presses of different capacities.
- Ceramic implants/samples machining facility.
- Electrical driers and furnaces with precision temperature and vacuum control.
- Infra-red, UV Vis and Raman spectroscopy, HPLC.
- Scratch tester.
- Micro-macro hardness tester.
- Planetary Mills.
Scientists
Scientists Profile
Dr. Vamsi Krishna Balla, Chief Scientist and Head
Dr. Sumana Das, Senior Principal Scientist
Dr. Biswanath Kundu, Principal Scientist
Dr. Mitun Das, Principal Scientist
Dr. Jui Chakraborty, Principal Scientist
Dr. Pradyot Datta, Principal Scientist
Dr. Subhadip Bodhak, Senior Scientist
Technical/Support Staff
| Name | Designation | Expertise |
| Debashish Bhattacharjee | Senior Technician (2) | CMC |
| Pradeep Kumar Patra | Technician (1) | Operation of Lathe machine |
Scholars/Students
| Student Name | Designation | Supervisor |
| Ms. Mita Biswas | Research Associate (RA) | Dr. Siddhartha Bandyopadhyay |
| Ms. Rupa Haldar | Research Associate (RA) | Dr. Siddhartha Bandyopadhyay |
| Dr. Somoshree Sengupta | DST Women Scientist | Dr. Vamsi Krishna Balla |
| Ms. Anuradha Jana | DST Women Scientist |
Dr. Vamsi Krishna Balla Dr. Mitun Das |
| Ms. Itishree Ratha | CSIR SRF | Dr. BiswanathKundu |
| Mr. Arnab Mahato | CSIR SRF | Dr. BiswanathKundu |
| Mr. Suman Saha | DST Inspire Fellow | Dr. Jui Chakraborty |
| Mr. Arnab Bhattachrya | SRF Project | Dr. Jui Chakraborty |
| Sk. Hasanur Rahman | Project Fellow | Dr. Jui Chakraborty |
| Ms. Payal Roy | CSIR JRF | Dr. Jui Chakraborty |
| Mr. Tarun Jana | MTech student (jointly with JU) | Dr. Jui Chakraborty |
| Dr. Aniruddha Pal | DST-National Postdoctoral Fellow | Dr. Subhadip Bodhak |
| Ms. Swarnima Agarwal | MTech student (jointly with IIEST) | Dr. Subhadip Bodhak |
Contact
Dr. Vamsi Krishna Balla
Chief Scientist and Head
Phone: +91-(033) 2322 3219
Fax: +91-33-24730957
E-mail: vamsiballa@cgcri.res.in
Last Updated on July 9, 2021









