English | हिन्दी

সিএসআইআর-কেন্দ্রীয় কাঁচ ও সেরামিক গবেষণা সংস্থা
सीएसआईआर-केंद्रीय काँच एवं सिरामिक अनुसंधान संस्थान
CSIR-Central Glass & Ceramic Research Institute
"Innovation in Ceramics and Glass for the mankind"

Biomaterials and Medical Devices
Overview
The Biomaterials & Medical Devices Division is a multidisciplinary research group whose mission is to develop materials, devices, implants and approaches for biomedical applications. Our research activities are targeted to provide state-of-the-art healthcare to all segments of Indian population primarily at ‘affordable cost’. Our technologies are aligned to national missions such as Swasthya Bharat, Make in India and Innovate in India. The division is among few forerunners in the country involved in developing materials and devices for biomedical applications.
We strongly believe in collaborative research and through effective alliance programs, that include close association of scientists, technologists, surgeons and industries, we actively work on research project that translate into effective healthcare products. With this ideology our research group has developed ceramic femoral head articulating against standard polyethylene of hip joint prosthesis. This product has been clinically tested followed by commercialization. Currently, we are working on new ceramic matrix composite for ceramic-on-ceramic articulating system for Total Hip Arthroplasty. To improve osseointegration of metallic implants we have developed bioactive hydroxyapatite (HAp) coating using plasma spraying and these coatings have been tested clinically. Other commercial biomedical products developed by the division include bioglass coatings, bioactive ceramic scaffolds with controlled porosity characteristics, calcium phosphate granules (single and biphasic) for dental and orthopaedic applications and hydroxyapatite based integrated orbital implant. Other important materials for biomedical applications that are currently under development include ceramic composites, hard coatings, bioglass and nanopowder for drug delivery. In addition to healthcare section, our division is developing ceramic components such as high-temperature, non-shrinkable ceramic potting material, freestanding diamond windows and diamond rods for supports in helix traveling-wave tube (TWT), as a part of India’s contribution towards International Thermonuclear Experimental Reactor (ITER) program.
The division has developed 11 technologies, commercialized 9 technologies and received two best technology awards from Government of India. We currently hold 23 patents including 2 USA and Japan patents. Currently, the division has research funding of ₹ 489 million (USD 7.69 million) during 2012 – 2018 and the divisional members include 7 scientists, 8 technical staff, 17 students (10 Ph.D.; 2 M.Tech; 5 Project staff).
1) Bioactive glass woundcare matrix for healing of diabetic ulcer (clinical trials are being initiated with industry support)
2) Electrospun composite dressing mat made of collagen derived from sweet water fish-skin waste and novel ion doped bioactive glass for the treatment of various wounds including normal, diabetic and burn (Animal trial completed)
3) Novel ion doped bioactive glass and egg-shell membrane (derived from egg-waste) composite as wound-dressing material for scar free wound management. (Animal trial completed)
Areas of Research
Due to our extensive collaboration throughout the country and abroad, our research activities include:
- Orthopaedic, dental and maxillofacial implants/materials
- Ceramics and ceramic composites for hip and knee prosthesis.
- Metallic and ceramic implants for finger joints, oncology and spine replacement.
- Bioresorbable materials for bone replacement.
- Bioactive glass and ceramic based cements/materials.
- Tissue engineering & drug delivery
- Bioactive, porous materials for orbital implants and wound dressing applications.
- Tailored/designed scaffolds for onsite drug delivery.
- Nanoceramic based inorganic drug delivery systems for cancer therapy.
- Multifunctional nanofibrous scaffolds based system for cartilage regeneration.
- Bioactive glass based dressings for hemostasis/wound healing
- Reconstructive and Trauma materials
- Tailored/designed scaffolds for orthopaedic, cosmetic, cranioplasty reconstruction.
- Patient specific and customized implants via additive manufacturing.
- Coatings
- Hard, wear resistance and biocompatible metallic, ceramic and composite coatings for load-bearing implants.
- Bioactive, osteoconductive and osteoinductive coatings on bioinert medical materials.
- Porous coatings for bone tissue ingrowth.
- Microwave plasma chemical vapor deposition of diamond coatings.
- Other materials and areas
- Ceramic and polycrystalline diamond materials for electron tubes.
- Ceramic joining.
- Microwave processing of ceramic materials.
- Unconventional cancer and wound treatment.
Collaboration
India
- Indian Institute of Technology, Madras.
- Indian Institute of Technology, Kanpur.
- Indian Institute of Technology, Delhi.
- Indian Institute of Science, Bangalore.
- Indian Institute of Technology, Kharagpur.
- Vellore Institute of Technology, Vellore.
- National Institute of Technology, Suratkal.
- Malaviya National Institute of Technology, Jaipur.
- West Bengal U of Animal & Fishery Sci, Kolkata.
- Sancheti Inst. for Orthop. & Rehabilitation, Pune.
- Tata Memorial Hospital, Mumbai.
- Jubilant Kalpataru Hospital, Barasat.
- Disha Eye Hospitals and Research Centre (P) Ltd., Barrackpore, West Bengal.
- Sri Sankaradeva Nethralaya, Guwahati.
- Saijyothi Eye Institute, Secunderabad.
- Sir Ganga Ram Hospital, New Delhi.
- Wockhardt Medical Centre, Kolkata.
- R.G. Kar Medical College & Hospital, Kolkata.
- Burdwan Medical College & Hospital, Burdwan.
- Calcutta Medical College & Hospital, Kolkata.
- Regional Institute of Ophthalmology, National Medical College and Hospital, Kolkata.
- N.R.S. Medical College and Hospital, Kolkata.
- Sambhu Nath Pandit Hospital, Kolkata.
- Calcutta Medical Research Institute, Kolkata.
- All India Institute of Medical Sciences, New Delhi.
- Maulana Azad Dental College & Hospital, New Delhi.
- Dr. Rafi Ahmed Dental College & Hospital, Kolkata.
- M/s General Surgical Company (India) Pvt Ltd., Chennai.
- M/s Orthotech, Gujarat.
- M/s Mu Biomagg, Gujarat.
Foreign
- Medical University of Vienna, Austria.
- Washington State University, USA.
- University of Louisville, USA.
- A.M. Prokhorov Gen. Phys. Inst., Russia.
- University of Aveiro, Portugal.
- University of Turku, Finland.
- Abo Academy University, Finland.
- Univ. of Vigo, Spain.
- Mindanao State Univ., Philippines.
- Universität Bremen, Germany.
Technology Developed
 1. Ceramic based total hip prosthesis (ceramic-on-PE). |
 2.Plasma spray hydroxyapatite coating on metallic biomedical implants. |
|||
 3. Manufacture of bioactive ceramic scaffolds with controlled porosity characteristics. |
 4. Calcium phosphate granules (single and biphasic) for dental and orthopaedic applications. |
|||
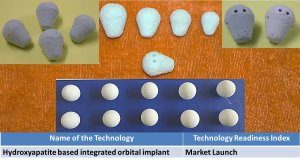 5. Hydroxyapatite based integrated orbital implant. |
 6. High-temperature protective coatings for automobile heater plugs. |
|||
 7. Glass lining coatings. |
 8. High-temperature protective coatings. |
|||
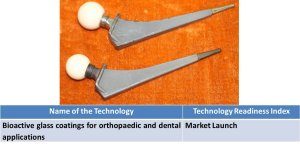 9. Bioactive glass coatings for orthopaedic and dental applications. |
 10. Plasma spray grade hydroxyapatite powder preparation. |
|||
 11. Injectable, biodegradable bone cement composite with/without drugs. |
12.”Multi-ion doped hydroxyapatite for dental and orthopedic applications’. Status: Pre-clinical studies completed. 13. Development of a novel ceramic based antacid compound for the treatment of hyperchlorhydria. Status: Preclinical studies completed 14.“Useful trace elements containing hydroxyapatite/ β-tricalcium phosphate from biogenic resource, Status: Pre-clinical studies completed. |
Technologies under development
| Sl. No. | Title | Technology Readiness Index |
| 1 | An inorganic/ceramic based antacid for instant relief and sustained buffering action. | Lab Validation |
| 2 | Novel HAp coated bone plates for bone plate fracture | Lab validation |
| 3 | Hydroxyapatite-bioactive glass composite for spinal and dental applications | Lab validation |
| 4 | Laser assisted deposition of in-situ synthesized TiB-TiN reinforced Ti alloy composites for biomedical and other applications. | Prototype |
| 4 | Metacarpophalangeal joint replacement prosthesis | Prototype (under clinical trials) |
| 6 | Alternative adjuvant for anticancer drugs | Proof of Concept |
| 7 | Unconventional cancer treatment without drugs | Proof of Concept |
| 8 | New ceramic composite composition for total hip prosthesis | Concept Definition |
| 9 | Non-invasive acceleration of tissue-artificial implant integration | Concept Definition |
| 10 | Porous metal coatings on metals and plastics for osseointegration | Concept Definition |
| 11 | Novel degradable Mg alloys with tailored resorption | Concept Definition |
| 12 | Patient specific customized implants | Concept Definition |
| 13 | Extendable implants for juvenile bone cancer patients | Concept Definition |
| 14 | Electrospun fiber composites for bone or skin wound healing | Concept Definition |
| 15 | Plasma-sprayed bioactive ceramics coated polymeric implants for spinal fusion surgery | Proof of Concept |
| 16 | Development of sugar-glass based nanocarriersystem for sustainable therapeutic protein delivery | Proof of Concept |
| 17 | Development of borate based bioactive glass micro/nanofibre for the treatment of non healing diabetic wound | Proof of Concept |
Technology Readiness Index: Idea, Concept Definition, Proof of Concept, Prototype, Lab Validation, Technology Development, Technology Demonstration, Technology Integrated, Market Launch.
Projects
(A) On-going Divisional Projects:
| Sl. No | Title of the Project | Duration | Project Category | Funding Agency | Principal Investigator | Co-Investigator(s) & members |
| National Projects | ||||||
| 1 | Oxidation and hot corrosion study on functionally graded multilayer thermal barrier coating for gas turbine applications | 2018-2021 | GAP | AR&DB | Dr. Sumana Ghosh | Dr. Mitun Das |
| 2 | Additive Manufacturing by Laser Metal Deposition | 2018-2019 | OLP | TATA Steel | Dr. Vamsi Krishna Balla |
Dr. Mitun Das Dr. Pradyot Datta |
| 3 | Wear resistant ceramics for cutting & milling operation: Process optimization of SiAlON-WC composites for rock drilling application | 2018-2020 | FTT | CSIR | Dr. Siddhartha Bandyopadhyay | Dr. SoumyaSarkar |
| 4 | Sugar-Glass Nanoparticles Encapsulated Multifunctional Nanofibrous Patch For Intervertebral Disc Regeneration | 2018-23 | GAP | DBT (RamalingaswamiReentry Fellowship) | Dr. Subhadip Bodhak | None |
| 5 | Preparation of high Heat Resistant CG-B-55A enamel frit for application in MiG series aero-engines parts | 2017-19 | SSP | HAL | Dr. SumanaGhosh | Dr. Jui Chakraborty Member: Dr. SoumyaSarkar |
| 6 | Preparation of high Heat Resistant CB-ABK-13 enamel frit for application in MiG series aero-engines parts | 2017-19 | SSP | HAL | Dr. SumanaGhosh | Dr. SoumyaSarkar Member: Dr. Jui Chakraborty |
| 7 | Microstructurally designed in-situ toughened metallic and ceramic matrix composites for total hip arthroplasty | 2016-19 | GAP | DBT | Dr. Vamsi Krishna Balla | Dr. Mitun Das, Mr. S. Gangadharan Member: Dr. Subhadip Bodhak, Dr. BiswanathKundu, Debashish Bhattacharya, PradeepPatra |
| 8 | Prototype Development of in vivo tested HDPE based biocompatible composite with HA/Al2O3 Ceramic Fillers as Acetabular Cup for Total Hip Replacement | 2015-20 | GAP | DBT |
Dr. BikramjitBasu (IISc), Dr. Vamsi Krishna Balla (CGCRI) |
Dr. BiswanathKundu Member: |
| 9 | The prospect of cancer therapy using an inorganic nano conjugate comprising microRNA-34 family | 2017-20 | GAP | DST | Dr. J. Chakraborty | Members: Dr. S. Ghosh, Dr. S.Bodhak, Dr. S.Sarkar |
| 10 | Development of hydroxyapatite based modified integrated orbital implant with superior motility and its clinical trial | 2013-16 | SBMT | CSIR-CGCRI | Dr. BiswanathKundu | Dr. Vamsi Krishna Balla |
| 11 |
Development of Minimally Invasive Bioactive Glass Nanoparticles Reinforced Injectable Hydrogel for Bone Tissue Engineering Applications |
2019-2021 | GAP264 | SERB-DST, India | Dr. Aniruddha Pal | Dr. Subhadip Bodhak (Mentor) |
| 12 | Synergistic Inhibitory Effect of Non-invasive Physical Stimulation on Cancer: A Promising Approach of Treatment | 2017-2020 | GAP260 | DST, India | Dr. Somoshree Sengupta | Dr. V. K. Balla (Mentor) |
| 13 | Biodegradable magnesium alloys with tailored degradation for bone replacement applications | 2017-2020 | GAP259 | DST, India | Anuradha Jana | Dr. V. K. Balla (Mentor) |
| International Projects | ||||||
| None at this point | ||||||
(B) Completed Projects
| Sl. No | Title of the Project | Duration | Funding Agency | Participating Inst./Lab. | Principal Investigator | Co-Investigator(s) |
| National Projects | ||||||
| 1 | Laser based additive manufacturing of in situ formed discontinuously reinforced Ti-TiB composite structures | 2016-19 | GAP | AR&DB | Dr. Mitun Das | Dr. Vamsi Krishna Balla |
| 2 | SiAlON inserts for High Speed Cutting of Hard Materials | 2016-18 | FTT | CSIR | Dr. S. Bandyopadhyay | Member: Dr. S. Sarkar |
| 3 | Development of novel ion doped hydroxyapatite by spray drying method and its utilization for plasma spray coating on medical implants with/without ion doping | 2016-18 | FTT | CSIR | Dr. B. Kundu | Dr. V. K. Balla Members: D. Bhatterchery, S. Majumder |
| 4 | Development of novel CSIR technology for manufacturing tailored and patient-specific bio-ceramic implants and biomedical devices at affordable cost (BIOCERAM) | 2012-17 | CSIR | Dr. V.K. Balla (coordinator) | S. Gangadharan, Dr. J. Chakraborty, Dr. B. Kundu, Dr. M. Das | |
| 5 | Structure of gradient nanocomposites: Interaction of bioactive glasses with nanoparticles and polymers (MoreBAGS) (GAP 0614) | 2012-16 | DST | Dr. G. De (CGCRI), Prof. PekkaVallittu (University of Turku, Finland) and Dr. LeenaHupa (ÅboAkademi Univ.) | Dr. B. Kundu, Dr. V.K. Balla | |
| 6 | Very High Power Microwave Tubes: Design and Development Capabilities (MTDDC) | 2012-16 | CSIR | Dr. S. Ghosh | A.K. Mallick | |
| 7 | Development of Novel Biomedical Implants with Enhanced Reliability | 2013-16 | DST | CSIR-CGRI | A.K. Mukhopadhyay | V.K. Balla |
| 8 | Comparative study of conventional processing with microwave processing of bioactive glass-ceramic coating on Ti6Al4V substrate for biomedical applications | 2013-16 | DST | Dr. S. Ghosh | ||
| 9 | Up-gradation of gamma radiation facility and periodic standardization for optimal RISUG production | 2015-17 | ICMR | B. Kundu | V.K. Balla | |
| 10 | Development of bio-ceramic based implants for rehabilitation | 2008-13 | CSIR | CSIR-CGCRI; SIOR & PHS, Pune | D. Basu& V.K. Balla | M. Das |
| 11 | Design and fabrication capabilities for very high power microwave tubes | 2007-12 | CSIR | CSIR-CGCRI & CSIR-CEERI | D. Basu | S. Datta |
| 12 | New insights in cancer biology identification of novel targets and development of target based molecular medicine | 2007-12 | CSIR | CSIR-CGCRI & CSIR-IICB | M.K. Sinha | B. Kundu |
| 13 | Nanomaterials and nanodevices in health and disease | 2007-12 | CSIR | CSIR-CGCRI & CSIR-CCMB | D. Basu | M.K. Sinha, Jui Chakraborty |
| 14 | Development of bioactive ceramic coating on orthopedic implants (metallic) for sustained, localized delivery of biophosphonates to improve fixation | 2009-11 | DST | CSIR-CGCRI | Jui Chakraborty | D. Basu |
| 15 | Synthesis and characterization of NZP based material for immobilization of radioactive waste | 2008-11 | BRNS (DAE) | CSIR-CGCRI | D. Basu | A. Das Gupta |
| 16 | Development of Ceramic-based Implantable Delivery System for Sustained Release of the Drugs for the Treatment of Osteomyelitis in Human Patients | 2007-10 | DST | CSIR-CGCRI, WBUAFS & R.G. Kar Medical College | B. Kundu | D. Basu |
| 17 | Fabrication of Yttria Doped Thoria (YDT) Thimbles and glass soldering them to Fe-Ni alloy component towards development of oxygen sensor for use in sodium coolant of fast breeder reactors | 2006-09 | IGCAR | CSIR-CGCRI | A. Das Gupta | D. Basu |
| 18 | Micromechanical characterization of hydroxyapatite coated metallic implants | 2006-09 | DST | CSIR-CGCRI | D. Basu | A.K. Mukhopadhyay |
| 19 | Development of selected medical implants (NMITLI) | 2005-08 | CSIR | CSIR-CGCRI | D. Basu | M.K. Sinha |
| 20 | Development of Glass-Ceramic Coatings for High Temperature Protection of Titanium Aluminide Alloys | 2005-08 | ARDB | CSIR-CGCRI | S. Datta | D. Basu |
| 21 | Multi-centred clinical trials for human implantation of bio-active integrated orbital implants | 2005-08 | ICMR | CSIR-CGCRI | D. Basu | B. Kundu |
| 22 | Development of bio-active bio-glass compositions with varied amount of calcium phosphate crystals to be used as coating on titanium and stainless steel implants | 2005-08 | DST | CSIR-CGCRI | S. Datta | D. Basu |
| 23 | Electron Tube Technologies for Large Scale Application | 2002-07 | CSIR | CSIR-CGCRI & CSIR-CEERI | D. Basu | S. Datta |
| 24 | Novel Synthetic Route for Biomaterials and their Applications | 2002-07 | CSIR | CSIR-CGCRI | D. Basu | M.K. Sinha |
| 25 | Developing Capabilities in Advanced Manufacturing Technology | 2002-07 | CSIR | CSIR-CGCRI & CSIR-CMERI | D. Basu | S. Datta |
| 26 | Development of plasma sprayed hydroxyapatite coated metallic implants for orthopaedic application | 2003-06 | DST | CSIR-CGCRI | D. Basu | M.K. Sinha |
| 27 | Development of metallized alumina substrates for fabrication of thermoelectric coolers | 2003-05 | SSPL (DRDO) | CSIR-CGCRI | S. Datta | D. Basu |
| 28 | Development of Bio-active Integrated Orbital Implant | 2001-04 | SBMT | CSIR-CGCRI | D. Basu | M.K. Sinha |
| International Projects | ||||||
| 1 | Borate based bioactive glass nano-fibers for topical application for treatment of non-healing diabetic wounds | 2017-19 | GAP | DST | J. Chakraborty (CGCRI) Prof. Mona Marei, Head, Tissue Engineering Laboratories, Faculty of Dentistry, University of Alexandria, Egypt, |
Dr. Swapan Kr. Ghosh |
| 2 | Electron microscopy study of the degradation kinetics of porous bioactive glass based novel drug eluting implants (coating/3D scaffolds) as a function of hard tissue regeneration for treatment of osteoporotic fractures in elderly patients | 2015-18 | GAP | DST | J. Chakraborty (CSIR-CGCRI) Dr. Nina Daneu (Jozef Stefan Institute, Ljubljana, Slovenia) |
B. Karmakar |
| 3 | Development of large size polycrystalline CVD diamond material for optical windows and support rods in high power microwave tubes | 2015-18 | DST |
V.K. Balla (CGCRI), PI from Russia |
A.K. Mallik | |
| 4 | Next Generation Functional Implants via Laser Engineering Net Shaping (LENS™) | 2012-15 | CSIR | CSIR-CGCRI, W. M. Keck Biomedical Materials Research Lab, Washington State University, USA. | V.K. Balla | Mitun Das |
| 5 | Development of functionally graded patient specific orthopedic implants by rapid prototyping technique and their evaluation in-vitro & in-vivo | 2009-12 | DST | CSIR-CGCRI; Washington State University, USA | D. Basu | M. Das |
| 6 | Development of Thermally Sprayed Ceramic Based Coatings | 2007-10 | TIFAC, DST | CSIR-CGCRI; ARCI, Hyderabad; School of Mechanical & Production Engg., Singapore; Advanced Materials Research Centre, Malayasia; & Indonesian Institute of Sciences | D. Basu | M.K. Sinha |
| 7 | Study of Remodeling of bone-ceramic interface to assess cell growth kinetics as a function of composition and morphological modification of ceramic implant | 2007-10 | DST | CSIR-CGCRI; Dept. of Nanostructured Materials, Ljubljana, Slovenia | D. Basu |
M.K. Sinha Jui Chakraborty |
| 8 | Development of ceramic-based implantable delivery system for sustained release of the drugs for treatment of osteomyelitis and diabetes | 2007-10 | DST | CSIR-CGCRI; University of Aveiro, Portugal | D. Basu | B. Kundu |
| 9 | Development of SiAlON ceramics for tribological applications | 2006-09 | CSIR | CSIR-CGCRI; Turkey | D. Basu | S. Bandyopadhyay |
Divisional Facilities
1) In vitro tribological and electrochemical testing facilities (Nanovea, USA; Bio-Logic Science Instruments SAS, France):
Our tribological testing system is equipped with rotating and pseudo linear reciprocating type wear testing modules. The test setup also contains accessories to carry out tests in physiological fluid with precise temperature control to represent biological environment in the body. Ball-on-disc or pin-on-disc wear testing can be performed using tribometer with 3 mm and/or 6 mm diameter ball/pins rubbing against test samples. Normal loads up to 40N can be used for tests. Our tribometer instrument has been recently upgraded with tribocorrosion test accessories, which enable isolation of  contribution from mechanical wear and corrosion. Electrochemical testing setup has potentiostat, galvanostat, electrometer and frequency response analyzer (FRA) designed to perform several electrochemical research experiments. We extensively use this instrument for electrochemical performance evaluation, such potentiodynamic, electrochemical impedance analysis, of variety of materials and coatings we develop for biomedical applications. Recently, the instrument has been upgraded with tribocorrosion test accessories, which enable isolation of contribution from mechanical wear and corrosion
contribution from mechanical wear and corrosion. Electrochemical testing setup has potentiostat, galvanostat, electrometer and frequency response analyzer (FRA) designed to perform several electrochemical research experiments. We extensively use this instrument for electrochemical performance evaluation, such potentiodynamic, electrochemical impedance analysis, of variety of materials and coatings we develop for biomedical applications. Recently, the instrument has been upgraded with tribocorrosion test accessories, which enable isolation of contribution from mechanical wear and corrosion
- Electrospinning equipment (Super ES-1, M/s E-Spin Nanotech Pvt. Ltd., Kanpur, UP, India):
Electrospinning is a technique used for preparing polymer/ceramic/composites fibers (both nano and micro fibrous patches) using precursor polymer/ceramic solution subjected to high DC electric field. Typical potential difference is applied in the range of 5-25 kV and the distance between needle tip to collector is typically kept at 10-20 cm to obtain electrospunfibers.
- Incubator Shaker (Spac N Service, Kolkata, india)
Specifically designed for long term and stable continuous operation ideal for pharmaceutical or biotechnology research for storage, incubation or release studies. Incubators to provide condition for optimal growth of microbiological cultures. It consists of speed and temperature controller which is required for the individual experimental protocol. The Maximum speed of the rotator is 200 rpm within a temperature range in the region of 25-60°C.

- Ion chromatography (792 Basic IC, Metrohm AG, Herisau, Switzerland)
Ion chromatography is a chromatographic process that separates ions and polar molecules based on their affinity to the ion exchanger. Typically Metrosep Organic Acids‐250 (6.1005.200), Metrohm, Switzerland, separation column comprising polystyrene divinyl benzene copolymer with sulfonic acid groups as carrier material (column dimension: 250 × 7.8 mm). A thermostated conductivity detector, attached to the IC having a cell volume of 1.5 mL, measuring alternating current in the conductivity range 0‐1000 mS cm−1 at 1 kHz for detection of the trace level ions in the analyte viz., Arsenite and arsenate, borate, organic acid etc. Further, Metrosep A Supp 5 – 250/4.0 separation column comprising Polyvinyl alcohol with quarternary ammonium groups having column dimension of 250 x 4.0 mm with a maximum pressure of 15MPa. This column is used for the detection of nitrite, bromide, nitrate, and iodide, chloride, sulphate etc in trace amount.
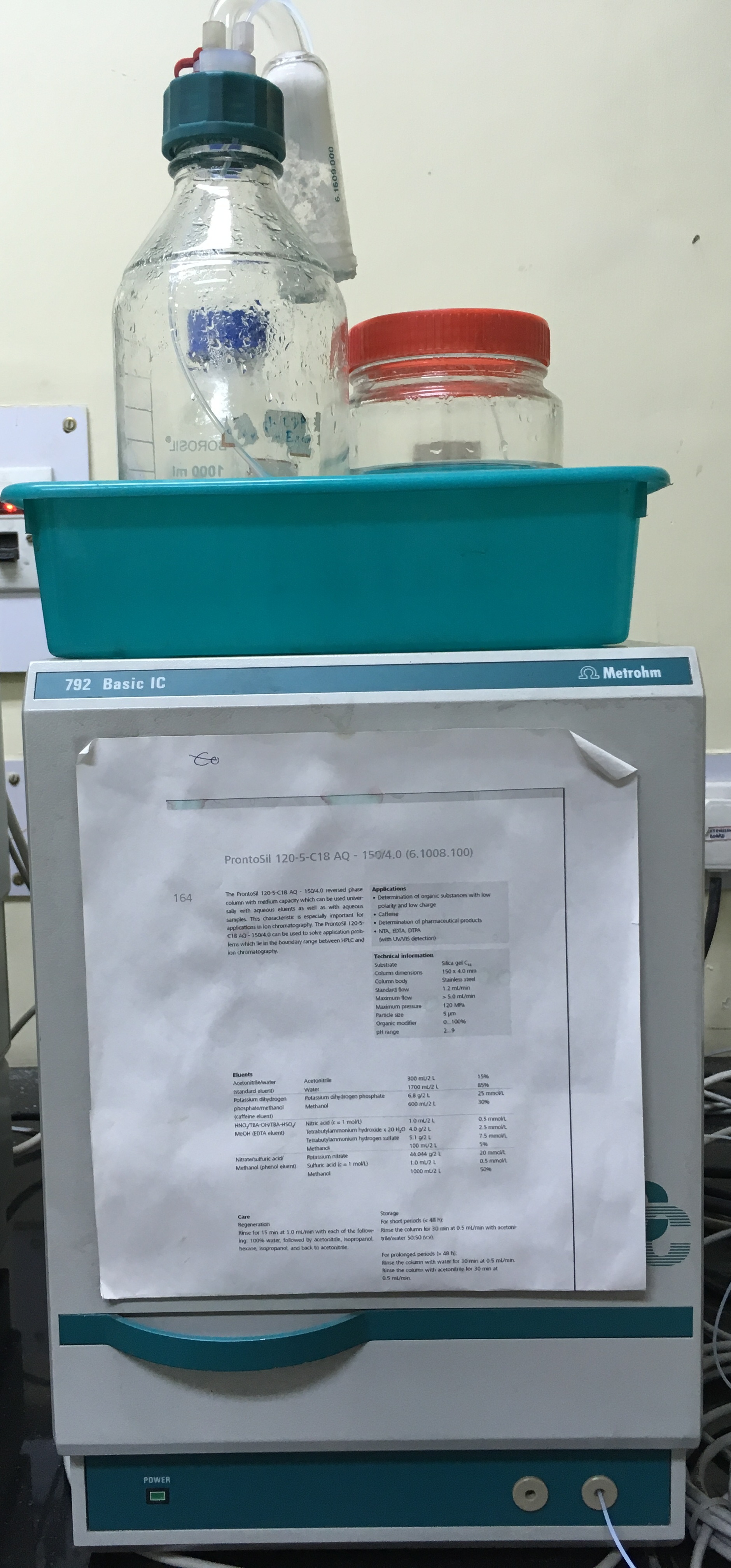
- Scratch Adhesion Tester (Ducom Instruments (Bangalore, India, TR-101-IAS)
There is a growing trend towards the use of modified surfaces for improved performance and life of components. Modifications are invariably in the form of coatings on base material. They are deposited by electroplating, vapor phase deposition, diffusion, thermal spray or welding. Coatings need to be characterized for parameters like scratch resistance, critical load, adhesion and nature of damage under high stress. The Scratch Tester by Ducom facilitates these measurements over a wide range of parameters. Start load in the range of 1 to 200N with a loading rate of 0-20N/sec. Maximum stroke length is 50 mm. Scratch speed in the range of 0.1-2 mm/sec and offset is in between 0.1-2 mm.

- Stability Chamber ( CSZ, Cincinnati Sub-Zero, OH 45241, USA)
Stability chambers are widely used in pharmaceutical laboratory, clinical trials, material testing and stability studies as per ICH guidelines. Test chamber is designed to create extremely stable temperature and humidity environments inside closed cabinet, a prerequisite condition to analyze the effects of pre-specified conditions w.r.t. temperature, humidity or both on life saving drugs and medicines, biological samples, electronic components and even industrial machine parts etc. This is suitable for stability studies including Long Term: 2-8°C, 25°C/60% RH, 25°C/40% RH, 30°C/35% RH or 30°C/65% RH, Intermediate: 30°C/65% RH and Accelerated: 40°C/75% RH, 25°C/60% RH.

Scientists
Scientists Profile
Dr. Vamsi Krishna Balla, Chief Scientist and Head
Dr. Sumana Das, Senior Principal Scientist
Dr. Biswanath Kundu, Senior Principal Scientist
Dr. Mitun Das, Senior Principal Scientist
Dr. Jui Chakraborty, Senior Principal Scientist
Dr. Pradyot Datta, Principal Scientist
Dr. Subhadip Bodhak, Principal Scientist
Mr. Ankush Pratap Singh, Scientist
Technical/Support Staff
| Name | Designation | Expertise |
| Birendra Kumar Tiwary | Technical Assistant | |
| Ayush Pandey | Technical Assistant | |
| Pradeep Kumar Patra | Technician (2) | Operation of Lathe machine |
| Pallab Mukherjee | Technician (1) |
Scholars/Students
| Student Name | Designation | Supervisor |
| Ms. Mita Biswas | Research Associate (RA) | Dr. Siddhartha Bandyopadhyay |
| Ms. Rupa Haldar | Research Associate (RA) | Dr. Siddhartha Bandyopadhyay |
| Dr. Somoshree Sengupta | DST Women Scientist | Dr. Vamsi Krishna Balla |
| Ms. Anuradha Jana | DST Women Scientist |
Dr. Vamsi Krishna Balla Dr. Mitun Das |
| Ms. Itishree Ratha | CSIR SRF | Dr. BiswanathKundu |
| Mr. Arnab Mahato | CSIR SRF | Dr. BiswanathKundu |
| Mr. Suman Saha | DST Inspire Fellow | Dr. Jui Chakraborty |
| Mr. Arnab Bhattachrya | SRF Project | Dr. Jui Chakraborty |
| Sk. Hasanur Rahman | Project Fellow | Dr. Jui Chakraborty |
| Ms. Payal Roy | CSIR JRF | Dr. Jui Chakraborty |
| Mr. Tarun Jana | MTech student (jointly with JU) | Dr. Jui Chakraborty |
| Dr. Aniruddha Pal | DST-National Postdoctoral Fellow | Dr. Subhadip Bodhak |
| Ms. Swarnima Agarwal | MTech student (jointly with IIEST) | Dr. Subhadip Bodhak |
Contact
Dr. Vamsi Krishna Balla
Chief Scientist and Head
Phone: +91-(033) 2322 3219
Fax: +91-33-24730957
E-mail: vamsiballa@cgcri.res.in
Last Updated on March 1, 2025









